2025-01-24
The Marketing Application of TUL』s Semaglutide Injection Accepted by the National Medical Products Administration
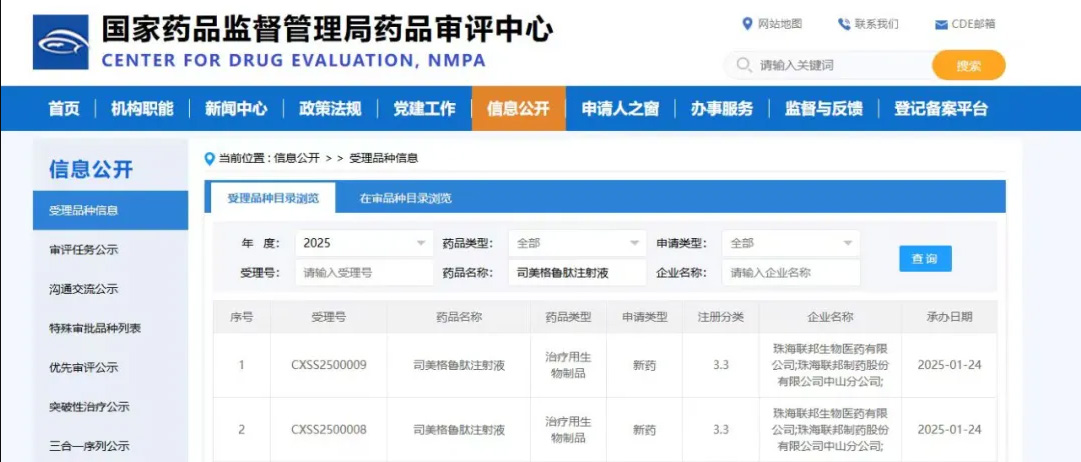
On January 23, 2025, the marketing registration application for Semaglutide Injection, submitted by Zhuhai United Bio-Pharmaceutical Co., Ltd., was officially accepted by the National Medical Products Administration (NMPA). This is the products variety declared by The United Laboratories following the company』s successful inclusion in the NMPA』s pilot program for segmented production of biological products.
Learn More